Synthesis, use and product manufacturing process of silicon carbide
Silicon carbide (SiC), also known as emery. In 1891, American Acheson (Acheson) invented the industrial manufacturing method of silicon carbide. Silicon carbide is synthesized using natural silica, carbon, wood chips, and industrial salt as basic synthetic raw materials, which are heated and reacted in a resistance furnace. The sawdust is added to make the block mixture porous at high temperature, so that a large amount of gas and volatiles generated by the reaction can be eliminated from it, and to avoid explosion, because the synthesis of IT silicon carbide will produce about 1.4t of carbon monoxide (CO). The role of industrial salt (NaCl) is to facilitate the removal of impurities such as alumina and iron oxide in the material.
1) Synthesis and use of silicon carbide
The synthesis of silicon carbide is carried out in a special resistance furnace, which is actually just a graphite resistance heating element, which is made of graphite particles or carbon particles stacked into columns. This heating element is placed in the middle. The above-mentioned raw materials are uniformly mixed in the proportions of silica 52%~54%, coke 35%, wood chips 11%, and industrial salt 1.5%~4%, and are tightly filled around the graphite heating element. When heated by electricity, the mixture undergoes a chemical reaction to produce silicon carbide. The reaction formula is:
SiO2+3C→SiC+2CO↑
The starting temperature of the reaction is about 1400°C, and the product is low-temperature β-SiC with very fine base crystals, which can stabilize to 2100°C, and then slowly transform to high-temperature α-SiC. α-SiC can be stabilized to 2400°C without significant decomposition. When it is above 2600°C, it sublimates and decomposes, volatilizing silicon vapor and leaving graphite. Therefore, the final temperature of the reaction is generally selected to be 1900~2200℃. The product synthesized by the reaction is a block-shaped crystalline polymer, which needs to be crushed into particles or powders of different sizes while removing impurities from them.
Sometimes in order to obtain high-purity silicon carbide, the method of vapor deposition can be used, that is, a mixed vapor of silicon tetrachloride, benzene and hydrogen is used. When passing through a hot graphite rod, a vapor phase reaction occurs, and the generated silicon carbide is deposited on the graphite surface. The reaction formula is:
6SiCl4+C6H6+12H2→6SiC+24HCl
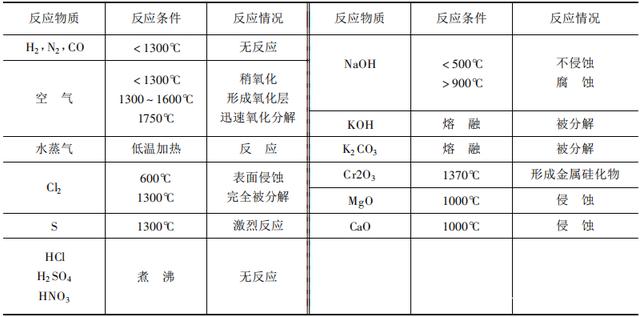
Pure silicon carbide is colorless and transparent. However, due to the presence of free carbon, iron, silicon and other impurities in silicon carbide produced in industry, the products have different colors such as yellow, black, dark green, and light green, and the common ones are light green and black. The relative molecular mass of silicon carbide is 40.09, of which silicon accounts for 70.04% and carbon accounts for 29.964. The true density is 3.21. Melting point (sublimation) 2600°C. The crystal type has a low-temperature form of β-SiC with cubic structure; high-temperature form of α-SiC has a hexagonal structure; and due to the difference in the arrangement of atoms in the silicon carbide crystal structure, there are other series of deformations, about a hundred kinds , Commonly known as isomorphous crystals. In addition, due to the difference in electron affinity in the crystal structure, in addition to the main covalent bonds, there are still some ionic bonds. Silicon carbide is a hard material with a Mohs hardness of 9.2. At low temperatures, silicon carbide has relatively stable chemical properties, excellent corrosion resistance, and is not corroded in boiling hydrochloric acid, sulfuric acid and hydrofluoric acid. However, it can react with certain metals, salts, and gases at high temperatures. The reaction conditions are listed in Table 10-4-16. Silicon carbide remains stable up to 2600°C in a reducing atmosphere, but oxidation occurs in a high-temperature oxidizing atmosphere:
SiC+2O2→SiO2+CO2
But its oxidation resistance between 800~1140℃ is not as good as that of 1300~1500℃. This is because the structure of the oxide film (SiO2) formed by oxidation at 800~1140℃ is relatively loose and cannot fully protect the substrate. The oxidation effect is significant above 1140°C, especially between 1300 and 1500°C. The oxide film formed at this time covers the surface of the silicon carbide substrate, hindering the further contact of oxygen to silicon carbide, so the oxidation resistance is Instead, strengthen. But at higher temperatures, the oxidation protection layer is destroyed, causing the silicon carbide to undergo strong oxidation and decomposition and destruction.
Because silicon carbide has excellent physical and chemical properties, it is widely used as an important industrial raw material. Its main uses are in three aspects: it is used to manufacture abrasives; it is used to manufacture resistance heating elements-silicon carbide rods, silicon carbon tubes, etc.; it is used to manufacture refractory products. As a special refractory material, it is used as a refractory product for stamping, corroded and worn parts of blast furnaces and iron furnaces in iron and steel smelting; it is used as smelting furnace lining and molten metal transportation in non-ferrous metal (zinc, aluminum, copper) smelting Pipes, filters, crucibles, etc.; used in space technology as rocket engine tail nozzles and high-temperature gas turbine blades; in the silicate industry, they are widely used as slats, muffle furnace linings, and cassettes for various kilns Bowl; In the chemical industry, it is used for oil and gas generation, petroleum gasifier, desulfurization furnace lining, etc.
(2) Product manufacturing process
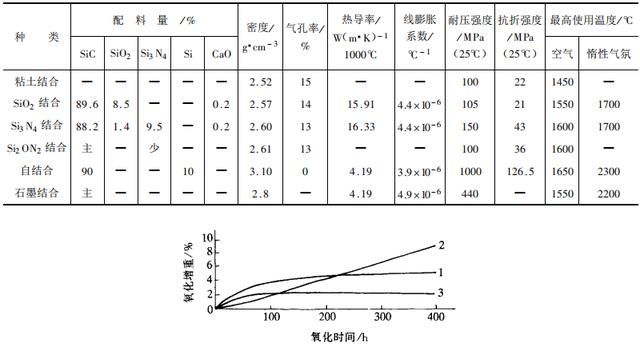
It is very difficult to grind it into micron-level fine powder because of its high hardness, and the particles are in the shape of plates or needles. The green body pressed by it will even be heated to its decomposition temperature. In the vicinity, significant shrinkage does not occur, it is difficult to sinter, the degree of densification of the product is low, and the oxidation resistance is also poor. Therefore, in the industrial production of products, a small amount of spherical β-SiC powder is added to α-SiC and additives are used to obtain dense products. As additives for product binders, it can be divided into oxides, nitrides, graphite, etc., such as clay, alumina, zircon, mullite, lime, glass, silicon nitride, silicon oxynitride, etc. , Graphite, etc. The forming binder solution can be one or more of carboxymethyl cellulose, polyvinyl alcohol, lignin, starch, alumina sol, silica sol, etc.
Depending on the type and amount of additives, the firing temperature of the green body is also different, and the temperature range is 1400-2300°C. For example, 70% α-SiC with a particle size greater than 44μm, 20% β-SiC with a particle size less than 10μm, 10% clay, and 8% 4.5% lignin aqueous solution, after uniform mixing, are molded with a pressure of 50MPa and placed in the air at 1400°C for 4h After firing, the volume density of the product is 2.53g/cm3, the apparent porosity is 12.3%, and the flexural strength is 30~33MPa. The sintering properties of the products with several different additives are listed in Table 2.
Generally speaking, silicon carbide refractories have excellent properties in many aspects, such as high strength, high thermal shock resistance, excellent wear resistance, high thermal conductivity, and chemical resistance in a relatively wide temperature range. Corrosion, etc. However, it should also be noted that its weakness is its poor oxidation resistance, which results in volume expansion and deformation at high temperatures that reduce its service life.
In order to improve the oxidation resistance of silicon carbide refractories, a lot of selection work has been done in terms of bonding agents. Initially, clay (including oxide) was used for bonding, but it failed to protect the silicon carbide particles. The silicon carbide particles were still oxidized and corroded. At the end of the 1950s, it was chosen to combine with silicon nitride (Si3N4) as an improved product of silicon carbide refractories. It does have good oxidation resistance (see Figure 1) without significant expansion. But the price is more expensive; in addition, there is the possibility of sudden damage when repeated heating and cooling; and the network structure of silicon nitride itself is permeable, which cannot fundamentally protect silicon carbide from being oxidized. In the early 1960s, silicon carbide refractories combined with silicon oxynitride (Si2ON2) appeared again, which had better oxidation resistance than silicon nitride combined because silicon oxynitride adhered to the silicon oxide on the surface of silicon carbide. Thin film, and react with it to form a continuous protective film firmly combined with silicon carbide. At the same time, the price of this material is appropriate, which is equivalent to a silicon carbide material combined with oxide.
Table 2 Properties of SiC products with different additives